When Recovery Turns Risky: Inside the Suboxone Lawsuits Shaking the Courts
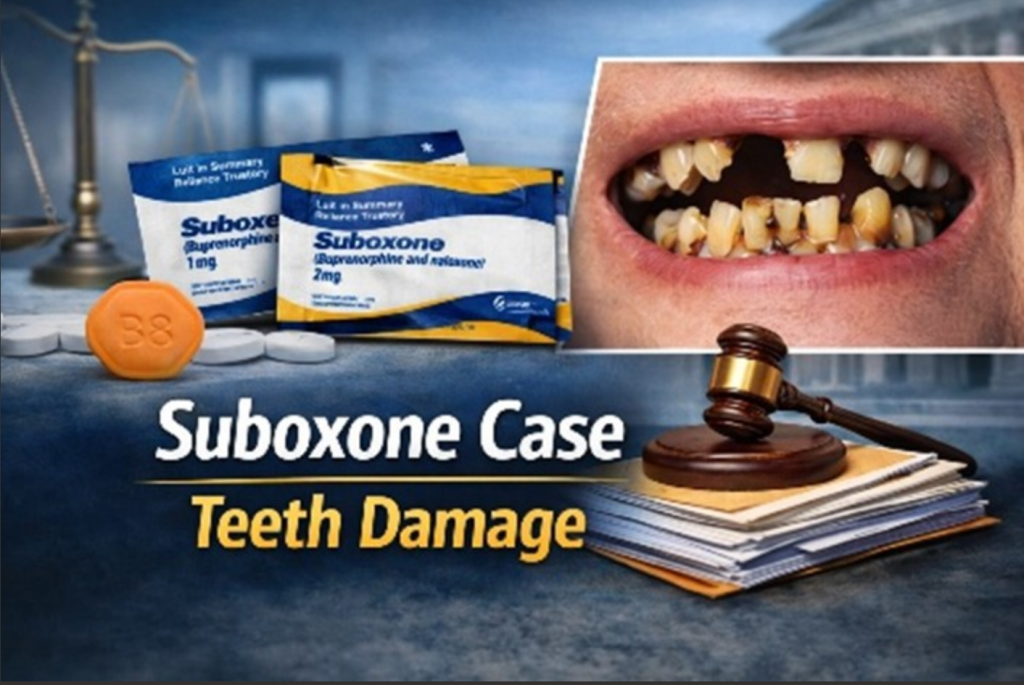
For years, Suboxone was hailed as a breakthrough – a medication that helped people break free from opioid addiction and rebuild their lives. But what started as a recovery success story has now turned into a major legal fight. Across the U.S., hundreds of patients are suing the drug’s maker, Indivior, claiming that Suboxone’s dissolvable film caused severe tooth decay and dental damage and that the company didn’t warn them in time.
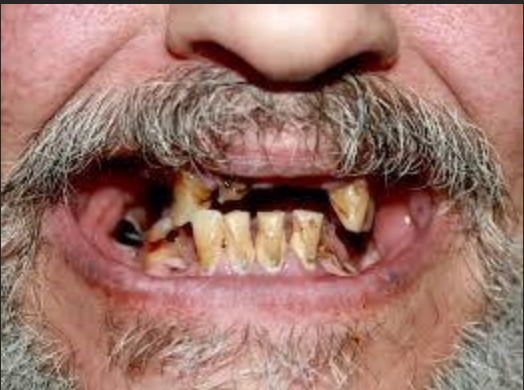
In January 2022, the U.S. Food and Drug Administration (FDA) released a public warning about Suboxone. The agency said it had received hundreds of reports of serious dental problems – like cavities, tooth loss, and infections from people using buprenorphine medicines that dissolve in the mouth, including Suboxone. What shocked many was that several patients had never had dental issues before. By June 2022, the FDA required manufacturers to add new warnings about dental risks to Suboxone’s prescribing information and patient Medication Guide. Soon after this alert, law firms across the country began filing lawsuits, claiming Indivior knew about these risks but failed to alert patients and doctors until the FDA made it public.
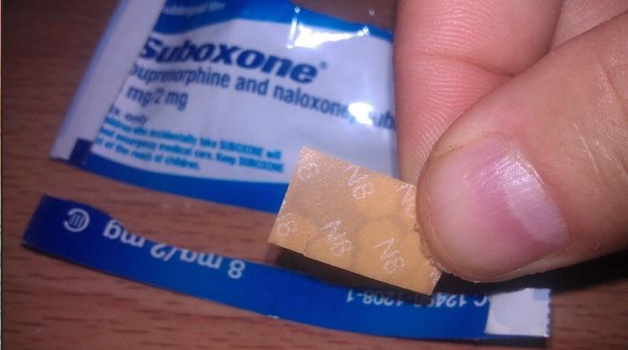
Most lawsuits make the same points. They claim Indivior:
- Didn’t warn about tooth decay and enamel damage
- Designed a product with an acidic film that harms teeth
- Marketed Suboxone as safe for long-term use without proper warnings
Indivior has denied these claims, saying it followed all FDA rules and that Suboxone’s benefits – especially in preventing opioid relapse – far outweigh its dental risks.
Because lawsuits were being filed nationwide, the courts grouped them together into one main federal case. In September 2023, the Judicial Panel on Multidistrict Litigation (JPML) created MDL No. 3092, called In re: Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation. The case is being handled in the U.S. District Court for the Northern District of Ohio by Judge J. Philip Calabrese. As of early 2026, there are about 1,854 active lawsuits. Both sides are now sharing evidence and expert opinions about what Indivior knew, when it knew it, and how quickly it acted after the FDA warning. No trial verdicts or settlements have been announced yet, but early test cases are expected soon.
Every mass tort begins with a warning and ends with lessons – legal, medical, and human. The Suboxone MDL will test how companies respond when side effects emerge years after approval. Whether the outcome is a settlement or a trial, it’s set to shape how future drug-safety warnings and corporate accountability are defined in court.
How Draft n Craft Can Help
Draft n Craft’s Medical-Legal Support team assists law firms handling drug injury and product liability cases like the Suboxone mass tort.
Our medical summarizers and experts help connect medical facts with legal strategy – turning complex patient data into clear, usable evidence.
| Service Area | What We Do | How It Helps in Suboxone-Type Cases |
| Medical Record Summarization | Review and condense medical and dental records into clear summaries | Quickly identify dental injuries, treatment history, and causation links |
| Chronology & Timeline Creation | Build detailed medical event timelines | Establish the sequence of Suboxone use and onset of dental decay |
| Injury Causation Analysis | Compare drug use patterns with reported injuries | Strengthen connection between Suboxone use and dental damage claims |
| Expert Review Support | Assist with preparing medical files for expert witnesses | Provide structured documentation for expert depositions or testimony |
| Plaintiff Fact Sheet (PFS) Support | Extract and organize data for MDL filings | Ensure accuracy and consistency across multiple claim submissions |
With precise medical summaries and structured reporting, Draft n Craft empowers attorneys to build stronger, faster, and more defensible claims in large-scale litigations like Suboxone.
Explore – Medico legal services/


