When Medical Devices Go Wrong: Inside the Bard PowerPort Lawsuits

Thousands of patients across the United States are suing medical-device manufacturer Bard Access Systems over its PowerPort implantable catheter, alleging that the device fractured, migrated, or failed inside the body, causing severe complications.
What began as individual lawsuits has now evolved into one of the most significant medical-device litigations in recent years; the Bard PowerPort Multidistrict Litigation (MDL No. 3081), officially titled In re: Bard Implanted Port Catheter Products Liability Litigation. The MDL is being overseen by U.S. District Judge David G. Campbell in Arizona and includes over 2,100 cases as of late 2025.

What the Bard PowerPort is and Why It’s Under Fire?
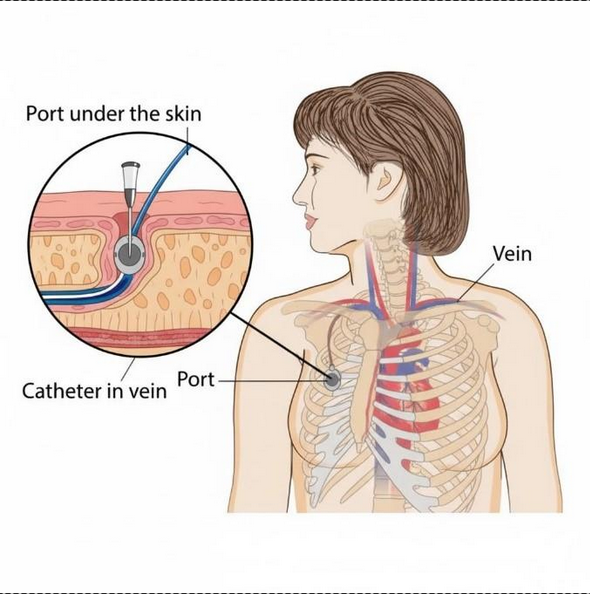
The Bard PowerPort is a small medical device implanted under the skin, primarily used for patients requiring long-term IV therapy or chemotherapy. It allows for easy and repeated access to veins.
Plaintiffs claim that due to design and material defects, the PowerPort’s catheter tube can crack or break apart, leading to:
- Blood clots (thrombosis)
- Infections
- Organ or vessel damage
Bard denies these allegations, arguing that failures may have been caused by surgical technique, patient-specific factors, or improper handling, not by a defective design.
Where the Litigation Stands
To manage the large number of lawsuits efficiently, the court has consolidated cases under the MDL system and established a bellwether trial schedule.
Those unaware, under Bellwether Trials – The court selects a few representative cases (the ones listed below) to go to trial first.
- These trials are “test cases”.
- The goal is to see how a jury reacts to key facts, evidence, and arguments.
- The outcomes give both sides insight into likely outcomes in the rest of the lawsuits.
These early “test” trials help both sides understand how juries might respond to key issues before hundreds of similar cases proceed. The first six bellwether plaintiffs are:
- Robert Cook
- Wanda Miller
- Judy Hicks
- Kimberly Divelbliss
- May Lattanzio
- Lloyd Sorensen
- (with Peter James as an alternate)
Trials are set to take place through 2026, and the outcomes could significantly influence settlement negotiations and future case strategies.

Key Legal Issues Shaping the Bard PowerPort MDL
1. What Really Caused the Device Failures
Central to the litigation is the question of causation:
Did the device fail due to a design or manufacturing defect, or were the complications caused by external factors like implantation technique or patient use?
Both sides are relying heavily on forensic testing, imaging, and metallurgical analysis of removed devices to prove their case.

2. What the FDA Records Reveal
The FDA’s adverse-event database (MAUDE) and recall history are major evidence sources.
Bard’s PowerPort devices were subject to Class II recalls in 2019, later terminated by the FDA.
- Plaintiffs argue these recalls show the company knew of systemic risks.
- Bard contends the recalls were limited in scope and that appropriate corrective actions were taken.
Patterns in adverse-event reports and internal complaint data could become pivotal in determining corporate knowledge and product safety.

3. The Preemption Debate
Another major question is federal preemption under the Medical Device Amendments (21 U.S.C. §360k).
If the PowerPort was approved through the FDA’s Premarket Approval (PMA) process, Bard could claim immunity from certain state-law claims.
If, however, it was cleared under the 510(k) pathway (based on substantial equivalence), preemption would not apply as strongly.
The litigation will likely hinge on the PowerPort’s regulatory classification and approval history.

4. Why Bellwether Trials Matter
The bellwether process will test not only jury reactions but also the admissibility and strength of expert testimony under Daubert standards.
- Strong verdicts for plaintiffs could push Bard toward a global settlement.
- Defense wins might limit the types of claims that move forward.
Either way, these trials will shape the valuation and future direction of the MDL.

What to Watch in 2026
The coming year will be critical. Attorneys and analysts are closely watching for:
- Early verdicts or settlements that set the tone for future negotiations.
- New disclosures or post-market studies that could confirm or refute systemic defect theories.
- Any new FDA actions or recalls, which could immediately impact litigation risk and strategy.

Why This Litigation Matters
The Bard PowerPort MDL highlights the complexity of modern product-liability litigation, where medical science, corporate records, and regulatory oversight intersect.
It demonstrates how multidistrict litigation (MDL) structures can balance efficiency with fairness, while revealing how device manufacturers and plaintiffs’ attorneys navigate the high-stakes battle between design accountability and regulatory compliance.

Draft n Craft: Supporting Complex Mass Tort & Product Liability Litigation
At Draft n Craft, we support attorneys through every stage of large-scale litigations, from medical record reviews and expert report summaries to data-driven discovery support for MDLs like Bard PowerPort, Paragard, and beyond.
Our dedicated paralegal teams combine precision, confidentiality, and litigation insight helping you stay ahead in complex, document-heavy cases.
Explore Litigation Support Services: https://www.draftncraft.com/know-your-edge

