GLP-1 Medications & NAION: What Patients and Lawyers Need to Know

Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) such as Ozempic, Wegovy, and related GLP-1 medications have rapidly become mainstream treatments for type 2 diabetes and obesity. Their appetite-suppressing and blood-sugar-lowering effects have made them among the most prescribed drugs worldwide. However, growing evidence suggests a rare but serious ocular risk: non-arteritic anterior ischemic optic neuropathy (NAION), a type of optic nerve stroke that can cause sudden, often permanent vision loss.
What Is NAION?
NAION is a vascular injury to the optic nerve resulting from insufficient blood flow. It typically presents as sudden, painless vision loss in one eye, often noticed upon waking. The condition can lead to partial or complete visual field loss and is generally considered irreversible once established.
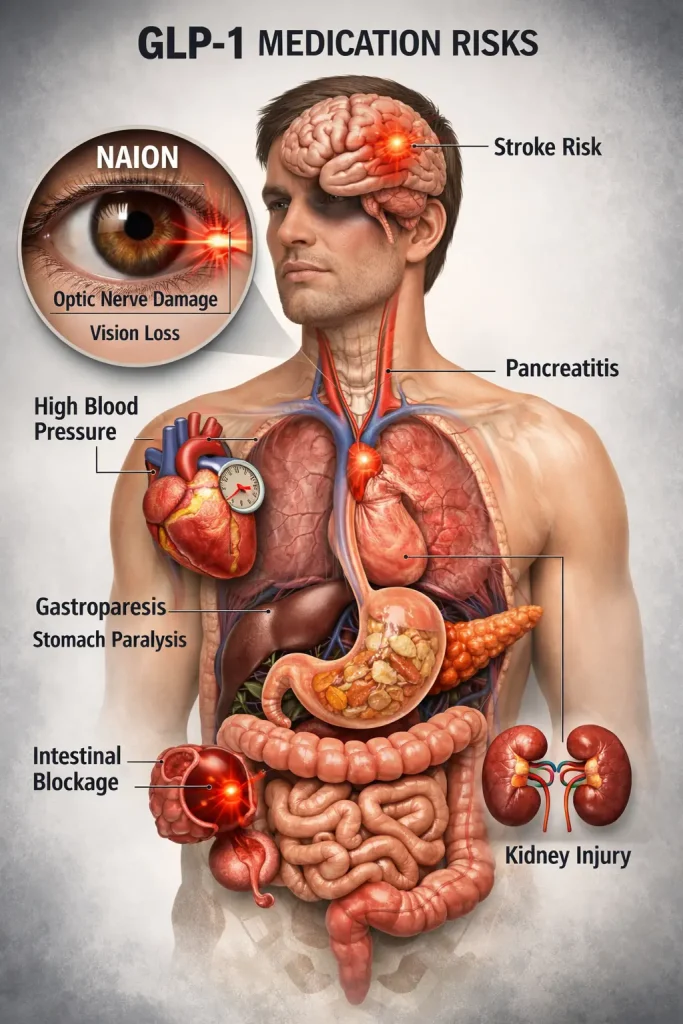
Emerging Evidence of Association With GLP-1 Drugs
Regulatory Review and Label Changes
In 2025, the European Medicines Agency (EMA) concluded its safety assessment of semaglutide medicines after concerns about NAION. Based on clinical trials, post-marketing surveillance, and observational data, the EMA determined that semaglutide may be associated with an increased risk of NAION albeit a very rare one (possibly affecting up to 1 in 10,000 users). The agency recommended updating product labels to reflect this potential risk.
Epidemiological and Clinical Studies
A 2025 meta-analysis pooled data from more than 1.5 million patients and found that GLP-1 receptor agonist use was associated with a modest but statistically significant increase in NAION risk (approximately 1.7-fold higher than in non-users). While the absolute risk remained low (0.09 %), the association was consistent across multiple study designs.
A large observational analysis of U.S. veterans with type 2 diabetes compared outcomes in those who initiated semaglutide versus sodium-glucose cotransporter-2 inhibitors (SGLT2is). The study found that semaglutide initiators had a 2.3-fold higher risk of incident NAION than those on SGLT2i therapy, with an absolute incidence rate of about 123 per 100,000 person-years (vs. 67 per 100,000).
Other retrospective cohort and case-control studies have also reported increased relative risks, in some analyses up to 4 – 7 times higher in semaglutide users compared with matched controls though absolute event counts remain small.
Risk Profile & Clinical Context
Rare but Serious
The data consistently show that NAION in GLP-1 users is rare but serious, with potential for permanent vision damage.
Biological Plausibility
While the precise mechanism by which GLP-1 agonists might contribute to NAION is not fully established, clinicians have hypothesized that rapid metabolic and hemodynamic changes combined with pre-existing risk factors like small optic disc size (“disc at risk”), hypertension, sleep apnea, and vascular disease, could exacerbate optic nerve ischemia in susceptible individuals.
Regulatory Advice
Healthcare professionals are advised to be alert for symptoms of sudden vision changes in patients on GLP-1 therapies, and patients are urged to seek immediate evaluation if such symptoms arise. Stopping the medication may be recommended if NAION is suspected or confirmed.
Legal Implications for Patients Harmed
Informed Consent and Duty to Warn
Clinicians and manufacturers have a legal duty to disclose known serious risks of medications. With increasing evidence linking GLP-1 drugs to NAION, inadequate warnings could be actionable in medical malpractice, product liability, or failure-to-warn cases.
Documentation Is Critical
Patients who experience sudden vision loss while taking a GLP-1 drug should preserve:
- Medical records and diagnosis of NAION
- Prescription history and dosing timeline
- Ophthalmologic evaluations and imaging
- Any communication with healthcare providers regarding vision symptoms
Causation & Expert Opinion
Because NAION may also occur in diabetes patients for other reasons, legal claims typically require expert testimony to connect the medication to the injury in a specific case. However, the growing body of epidemiological evidence strengthens the possibility of establishing such causation.
In Summary
- GLP-1 receptor agonists, including semaglutide products, are widely used for diabetes and weight management.
- Recent regulatory and clinical data suggest a very rare but real risk of non-arteritic anterior ischemic optic neuropathy (NAION).
- The absolute risk is low, but the consequences sudden, potentially permanent vision loss are severe.
- Plaintiffs harmed by NAION may have legal claims based on failure to warn, product liability, or medical negligence.
How Draft n Craft Supports Attorneys Handling GLP-1 & NAION Litigation:
| Stage | Litigation Need | What Draft n Craft Delivers | Attorney Benefit |
| 1. Medical Record Review & Chronology | Establish drug timeline & NAION diagnosis | • GLP-1 initiation verification • Symptom onset mapping • NAION confirmation • Risk factor extraction • Missing record flags | Clear causation narrative from Day 1 |
| 2. Causation-Focused Case Screening | Filter viable claims | • Intake validation calls • Prescription duration check • ICD diagnosis cross-check • Pharmacy & ophthalmology record organization • Screening summaries | Prevent weak claims from entering pipeline |
| 3. Mass Tort Workflow Management | Handle scale efficiently | • PFS preparation • Medical authorizations • Record retrieval tracking • Discovery coordination • Deadline monitoring dashboards | Operational control without internal hiring |
| 4. Expert-Ready Medical Bundles | Prepare cases for ophthalmology experts | • Structured medical timelines • Diagnostic imaging references • Risk factor summaries • Indexed & hyperlinked PDF sets | Faster expert review & reduced litigation cost |
| 5. Secure, Scalable DRP Model | Build long-term backend infrastructure | • Hire | Train |
Explore – Mass-Tort-Support-Solution/
